There is significant evidence that has been uncovered over the last ten years related to the existence of unusually high temperatures at the World Trade Center, both during the destruction of the three buildings and afterward for several months.
Much of the evidence has been catalogued in two peer-reviewed scientific papers. One of these papers is called “Extremely high temperatures during the WTC destruction,” and it was published online at the Journal of 9/11 Studies in January, 2008.294 The authors included four PhD physicists, one PhD chemist and several others, including me. The second of the two papers is called “Environmental Anomalies at the WTC, evidence for energetic materials,” and it was published both online and in print by a Springer journal called The Environmentalist in 2008.295 The authors include myself and two of my colleagues.
The first paper discusses the maximum temperatures that were cited by the National Institute of Standards and Technology (or NIST) at the WTC. In its report on the WTC destruction, NIST reported gas temperatures as high as 1000 °C. It is important to note that these are gas temperatures, not the temperatures of solid materials.
Others who have publicly supported the fire-induced collapse hypotheses for the WTC buildings, such as Professor Thomas Eagar of MIT, have suggested the same maximum temperature while still others have proposed that a slightly higher gas temperature of 1100 °C might theoretically have existed.
One problem with the maximum temperatures cited by officials is that there are many eyewitnesses who claimed to see molten metal at the WTC. Approximately 1000 °C cannot melt the steel in the WTC buildings. Just a few of the eyewitness statements regarding molten metal can be found below. The first one is from a man who worked for John Skilling, the design engineer of the WTC towers.
- There was a “river of steel flowing” at the B1 level of the WTC debris pile. — Leslie Robertson296
- “Going below, it was smoky and really hot… The debris past the columns was red-hot, molten, running.” — Richard Garlock, Structural Engineer / LERA
- “I talked to many contractors and they said they actually saw molten metal trapped, beams had just totally been melted because of the heat.” — Herb Trimpe, Chaplain at Ground Zero
- “[I was shown slides of] molten metal, which was still red hot weeks after the event.” — Dr. Keith Eaton, Institute of Structural Engineers
- “In some pockets now being uncovered they are finding molten steel.” — Dr. Alison Geyh, Johns Hopkins School of Public Health
- “[I] saw pools of literally molten steel.” — Peter Tully, president of Tully Construction
- “Feeling the heat, seeing the molten steel, the layers upon layers of ash, like lava, it reminded me of Mt. St. Helens… Shards of steel lay upon shards of steel, shifting and unstable, uncovering red hot metal beams excavated from deep beneath layers of subfloors.” — Ron Burger, structural engineer
- “A fire truck 10 feet below the ground that was still burning two weeks after the Tower collapsed, its metal so hot that it looked like a vat of molten steel.” — Vance Deisingnore, ASHA Officer at the WTC, reporting to Jim McKay, Post-Gazette Staff Writer, on Sept 11, 2002
- “I saw melting of girders in World Trade Center.” — Abolhassan Astaneh-Asi, the first structural engineer given access to the WTC steel via a National Science Foundation Grant
- “You get down below and you’d see molten steel — molten steel running down the channel rail, like you’re in a foundry, like lava.” — FDNY Fire Department Captain
NIST ignored all of these witness statements about molten metal in reporting maximum temperatures of 1000 °C.
There are also photographs that show bright orange and yellow molten metal pouring from the south tower, and being pulled from the debris pile at ground zero. NIST said that they found no evidence of molten metal, but also said if molten metal had been present, it would have had to have been aluminum from the plane. The molten metal could not have been molten aluminum, as molten iron or steel is yellow/orange, and molten aluminum is silvery gray when poured in daylight. Experimental demonstrations have been done to show this, which can be found at the Journal of 9/11 Studies.
The temperature required to melt steel (1538 °C) is far above the maximum gas temperature cited in the official report (1000 °C). In a structural fire, steel temperatures lag behind gas temperatures for a number of reasons, including the thermal conductivity of steel, the effects of convection, and the fireproofing that is applied. Achieving a steel temperature of 1538 °C at the WTC would require gas temperatures that are well above 1538 °C and far above the maximum of 1000 °C cited in the NIST report.
When the temperatures cited in the NIST report are achieved, for example in a testing furnace when the air temperature is raised quickly and held at 1,000 °C and heat is not conducted away by a large building structure, it takes approximately two hours for protected steel to reach just 600°C, which is still below the melting temperature of most forms of aluminum.297 And obviously the steel temperatures cannot exceed the gas temperatures in such an environment, to produce molten iron or steel. These facts demonstrate that the NIST reports do not address the evidence.
I have seen evidence of the previously molten metal at the WTC myself, in the form of metallic microspheres that I have found in all of the nearly dozen WTC dust samples I have examined. Photomicrographs of the first examples that I received, in 2007, are published online.298
I extracted the particles from a sample of WTC dust that had been given to me by someone who was at Ground Zero after the destruction of the buildings. Dr. Steven Jones of Brigham Young University had been examining WTC dust samples as well and I was interested in seeing for myself what he had seen.
After realizing that such findings could be used in a legal proceeding at some point in the future, my colleagues and I began asking that samples collected by these independent sources be accompanied by documentation that recorded the time, date and other necessary information including sample location. Each sample was provided with this information as well as the signature of the collector and sometimes a witness as well. The process evolved into the use of a standard chain of custody form similar to that which I have used for many years in my experience as a laboratory manager.
I have extracted the metallic microspheres and other paramagnetic particles from the dust in several ways. One way is to slide a magnet along the side of the bag containing the dust and capture what is attracted with a spatula. Another way is to place a stronger magnet into a plastic bag and insert that bag into the dust sample. Removing the bagged magnet and inverting the bag allows the particles to be captured.
It is interesting to note that the United States Environmental Protection Agency (or EPA) once considered using iron microspheres as a signature characteristic to identify WTC dust.299 For an unknown reason, EPA decided to not designate the iron spheres as a signature characteristic of WTC dust, despite the fact that it was known to be an unusual identifying characteristic of that dust.
The first paper mentioned above begins with a discussion of such metallic microspheres, as well as the finding of semi-transparent, silicate-rich microspheres. Two independently collected samples were received for this study. Both samples were collected indoors and shortly after the 9/11/2001 event. One sample was collected on an indoor window sill on 9/14/2001, just three days after the disaster while the search for survivors in the rubble was ongoing, in a building four blocks from ground zero. The other sample was acquired inside a fourth-floor apartment, whose upper windows broke during the WTC collapse, a few days later.
An important point to recognize is that the presence of these metallic and silicate microspheres, as well as much more such evidence, had already been reported by other independent researchers apart from the US EPA. The RJ Lee Group was one of those independent groups. RJ Lee is a corporation specializing in industrial forensics. It was hired by lawyers for Deutsche Bank to characterize the WTC dust as the Deutsche Bank building, located at Ground Zero, was being assessed after 9/11.300
RJ Lee produced a report that corroborates and expands upon the findings of our research group.
The second independent group to have corroborated our findings was the United States Geological Survey (USGS), which is a federal source for science about the Earth, its natural and living resources, natural hazards, and the environment. USGS coordinated an interdisciplinary environmental characterization of the entire area around the WTC after 9/11.
RJ Lee reported that the quantity of iron spheres in the WTC dust was 5.87%. This is an enormous amount relative to what is found in typical dust samples from office buildings. In fact, RJ Lee reported that it is 150 times as much.
The spherical shape of the particles indicates that they were at one time molten (liquid) metal. As with water falling or spraying through air, molten metal forms spheres due to surface tension. The cohesive forces between liquid molecules are responsible for this. When a liquid is falling or sprayed through the air, the molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface. This forces liquid surfaces to contract to the minimal area.
The WTC dust spheres indicate not only that the iron or silicate was molten at one point, but that, due to the small size of the spheres, a violent disturbance of some kind would have been necessary to shatter molten metal into the sizes seen. Various explosive or incendiary processes are likely explanations.
The RJ Lee report says that lead was melted and that such particles are absent in typical office dust.
“Various metals (most notably iron and lead) were melted during the WTC Event, producing spherical metallic particles. … high heat exposure of the WTC Dust has also created … spherical, vesicular siliceous [silicate particles] and [these]…are classic examples of high temperature or combustion by-products and are generally absent in typical office dust…”301
Surprisingly, these researchers also reported that alumino-silicates were evaporated at the WTC, as indicated by the Swiss cheese appearance of some of the particles examined in the dust.
RJ Lee also reported a “vesicular alumino-silicate particle” which exemplifies a “round open porous structure having a Swiss cheese appearance as a result of boiling and evaporation.”302
The United States Geological Survey found the same iron and silicate spheres throughout the WTC dust and could not find an explanation.
Two members of our research team submitted a Freedom of Information Act request to the USGS for any other information that might not have been reported. To our surprise, the USGS responded with data showing that their group had found molybdenum microspheres in the WTC dust. The presence of these molybdenum spheres indicates that there was molten molybdenum at the WTC site. The temperature required to melt molybdenum is 2,623 °C.
Our research team, led by Dr. Steven Jones and Dr. Jeffrey Farrer at Brigham Young University, analyzed the metallic microspheres we found in the WTC dust by a technique called X-ray Energy Dispersive Spectroscopy, or XEDS. This is a technique that provided the elemental composition of the spheres. In other words, it told us what elements the spheres were composed of. The result was that the spheres we found were very high in iron and low in other elements. This agreed with the findings of the RJ Lee group.
The discoveries related to these high temperatures continued. RJ Lee further reported that lead had not only melted, it had “volatilized.” That is, lead had actually vaporized at the WTC, according to the RJ Lee research report, which said: “The presence of lead oxide on the surface of mineral wool indicates the existence of extremely high temperatures during the collapse which caused metallic lead to volatilize, oxidize, and finally condense on the surface of the mineral wool.”
The RJ Lee report further stated: “Some particles show evidence of being exposed to a conflagration such as spherical metals and silicates, and vesicular particles.” A vesicular formation is a round open porous structure having a Swiss cheese appearance as a result of boiling and evaporation. These kinds of vesicular formations are abundant in particles extracted from WTC dust samples.
The most important point of all this is that the official US government investigators into the WTC disaster reported gas temperatures that were far lower than what would be required to explain these findings from RJ Lee, the USGS, and our research team.
As shown in Table 10-1 below, the temperatures required to melt iron, vaporize lead, melt molybdenum, and vaporize alumino-silicates give evidence for an environment at the WTC that was nearly two thousand degrees hotter than what official investigators have reported as maximum gas temperatures. TTT
Table 10-1: Temperatures required based on the evidence
| Process and material | °C | °F |
| To melt iron (spherule formation) | 1,538 | 2,800 |
| To vaporize lead | 1,740 | 3,164 |
| To melt molybdenum | 2,623 | 4,753 |
| To vaporize aluminosilicates | 2,760 | 5,000 |
More corroboration for these findings is found in the official US government report that preceded the current one published by NIST. The first report was from the Federal Emergency Management Administration (FEMA). Appendix C of the FEMA WTC report provided strong evidence of extremely high temperatures at the WTC, in the form of highly corroded and eroded steel samples saved from the buildings that had been destroyed.303
FEMA described samples of steel that had been thinned to razor-sharpness. In some cases there were inexplicable holes in the steel. The fire engineering professors who found these samples could not come up with an explanation for it. They also could not explain the sulfidation of the steel. That is, steel had been chemically changed at the micro-structural level in ways that indicated a chemical eutectic mixture had been achieved between sulfur, iron and oxygen, causing the steel to melt.
The New York Times called these findings “the deepest mystery uncovered in the [WTC] investigation.”304 That mystery has never been officially solved and the related evidence was completely ignored by NIST.
Other evidence for extremely high temperatures at the WTC site includes the finding of fused metal and concrete artifacts like the “meteorite” and also thermal hot spots measured by a NASA remote sensing instrument that measures temperature via electromagnetic radiation emitted from the ground. Surface temperatures in the debris piles were found to be as high as 750 °C (or 1350 °F) a week after 9/11.
There is an explanation available for all this officially unexplained evidence. This explanation is that the thermite reaction was present and occurring at the WTC on 9/11 and afterward, in the pile at Ground Zero. The thermite reaction is an extremely exothermic chemical reaction between aluminum powder and a metal oxide. The metal oxide is typically iron oxide but copper oxide, molybdenum oxide and vanadium oxide are also used, among others.
The temperature at which thermite burns approaches 3,000 °C for some mixtures, which would explain the evidence for high temperatures described above. The reaction products of an aluminum/iron oxide thermite mixture are molten iron, and aluminum oxide, which quickly forms a white dust cloud as it cools. Additives like sulfur improve the burn properties of thermite. A sulfur-containing thermite, which is called thermate, would explain the evidence found by the FEMA investigators.
The color of the molten iron product from thermite reactions is yellow-orange, just like the photos of molten metal witnessed at the WTC. The photographs of molten metal at the WTC, pouring from the south tower and found in the debris pile, exhibit the yellow-orange color, unlike molten aluminum, which is silvery gray when poured in the daylight.
Even though a thermite reaction is a good explanation for the molten iron, some have suggested that it is an innocuous explanation because there was aluminum in the planes and rusty metal (or iron oxide) in the buildings. Such claims suggest that innocent components of the buildings and planes coming together might have caused the thermite reaction to occur.
Unlike NIST, however, we actually tested that hypothesis. A colleague of Dr. Steven Jones poured molten aluminum over a rusty steel rail and found that the thermite reaction will not occur in that scenario. This was expected because thermite mixtures are powders mixed in an exact ratio and require a high temperature ignition device to ignite.
Of course, the “natural thermite” hypothesis would also fail to explain the molten molybdenum found by USGS and the RJ Lee group.
The second of the two peer-reviewed scientific articles referred to above focuses on air emissions data produced by EPA and the University of California Davis. Before going into the environmental data, the paper reviews some important facts about the environment at ground zero in the days, weeks and months after 9/11. The fires at ground zero could not be put out, and continued to burn in one place or another throughout the pile for months, even into February 2002.
This was despite the fact that:
- Several inches of dust covered the entire area after the destruction of the WTC buildings.
- Millions of gallons of water were sprayed onto the debris pile.
- Several rainfall events occurred at the site, some heavy.
- A chemical fire suppressant called Pyrocool was pumped into the piles, but had no effect.305
Such characteristics are not typical of structure fires and cannot be explained by typical office fire phenomena.
The EPA data discussed in the paper was released to my local investigative group, which is called the 9/11 Working Group of Bloomington. This data shows certain patterns of extreme emissions occurring at the WTC site. Figure 10-1 shows an example of those unusual patterns. These five chemicals, all of the type called volatile organic compounds or VOCs, exhibited spikes in detection on the same dates.
Figure 10-1: Spikes in detection of VOCs in air ground zero
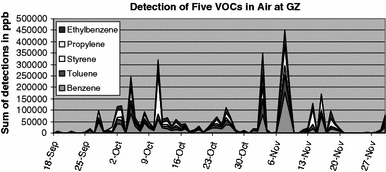
All of these compounds were emitted into the air at high levels on the dates given. These chemicals are the byproducts of the combustion of plastics, which often burn only partially in a fire.
The levels at which these VOCs were seen at the WTC site were unprecedented. As an example, consider that benzene has been seen at levels as high as 26 parts per billion (ppb) in structure fires. Benzene is also seen in high-traffic areas of urban settings, with mean levels of 4 ppb. At the WTC site, benzene was detected in bursts of 80,000 ppb and higher.
These VOC levels indicate that plastics and other organic materials were burning to completion and doing so very rapidly within the pile at ground zero.
Similar spikes in other chemical compounds were seen. Specifically, there were spikes in detection of iron, aluminum, and compounds of silicon and sulfur. There were also spikes in detection of rare metals, like vanadium, and an unusual synthetic organic chemical called 1,3-diphenylpropane (1,3-DPP).
The EPA noted that it had never before seen 1,3-DPP in any of its environmental testing. Erik Swartz, a research scientist at EPA, noted that 1,3-DPP was pervasive and was found at levels that “dwarfed all others.”306 One use of 1,3-DPP is to stabilize the structure of nanocomposite materials.307
The EPA findings were corroborated by aerosol data produced by a team from the University of California Davis near the WTC site in October 2001. The UC Davis data exhibited spikes in the detection of silicon compounds as well as aluminum and iron compounds.
When publishing their results, the UC Davis team noted several problems could not be explained. They reported as follows:
- We see very fine aerosols typical of combustion temperatures far higher than [expected in] the WTC collapse piles.
- We see some elements abundantly and others hardly at all, despite similar abundances in the collapse dust.
- We see organic species in the very fine mode that would not survive high temperatures.308
These data are compelling when one considers that there is a form of thermite that contains silicon compounds and organic materials. This material is sometimes referred to as nanothermite or superthermite. And although this essay does not go into the discovery of nanothermite at the WTC, which is discussed elsewhere in this volume, we should recognize two facts.
First, the compounds detected at the WTC site, in spikes and at extreme levels, indicate the presence of violent fires occurring on specific dates. These compounds also match well with sulfur-containing thermate and/or with nanothermite. Secondly, the official investigators are not willing to examine or even discuss these data.
I have made nanothermite myself, via formulations published by U.S. national research laboratories, and I have ignited that nanothermite. When we look at the ignition residues, they are strikingly similar in appearance to WTC dust particles that were extracted with a magnet. Both are the same colors, and show the same metallic microspheres. Both also exhibit the same kind of relative size and vesicular formations that suggest high temperature reactions or explosive effects.
There are two more important points of evidence relating to the high temperatures at the WTC site. The first is that the huge dust cloud that arose from the destruction of the buildings was similar to that of a volcano. In other words, it was pyroclastic-like and appeared to be driven by energy sources that exceeded the energy available from a simple gravitational collapse. Calculations by researcher Jim Hoffman, based on photographs of the size and distribution of the clouds, have confirmed that the energy is not accounted for by gravitational effects alone.309
Another striking fact is that the dust cloud was very hot and was burning people and setting objects on fire. In the public domain, there are photos of the many vehicles that were set on fire or burned in the area.
Paul Curran, a member of the New York City Fire Patrol, was asked what he thought was the cause of these vehicle fires. Curran responded, “I believe it must have been from the debris falling and the heat just started hitting the cars and starting cars on fire. There were an awful lot of cars burning, an awful lot. It had to be radiated heat or just stuff falling on cars and setting them on fire. There were numerous cars burning, numerous.”
There were also many witnesses to the cloud being very hot and burning people as it passed by. The following are excerpts from some of their testimonies:
- “Then the dust cloud hits us. Then it got real hot. It felt like it was going to light up almost.” — Thomas Spinard, FDNY Engine 7
- “A wave — a hot, solid, black wave of heat threw me down the block.” — David Handschuh, New York’s Daily News
- “…the hot billowing cloud of death chasing us through the narrow streets of lower Manhattan” — Andred Fagan
- “When I was running, some hot stuff went down my back, because I didn’t have time to put my coat back on, and I had some — well, I guess between first and second degree burns on my back.” — Marcel Claes, FDNY Firefighter
- “I was running, and stuff was coming down. This time fire was coming down, because I could feel the heat. I grabbed a firefighter’s turnout coat that just seemed to be in front of me. I grabbed it. I threw it over my shoulders. I didn’t make it much further than that. …It was really hot, because this time there was fire. I know that because my neck burned.” — Louis Cook, FDNY Paramedic Division
- “By the time it took me to break the back window of the SUV my safety coat was already on fire, my socks were on fire.” — Ronald Thomas Coyne, EMT Battalion 44
- “Sal ran west somewhere and got blown off, got burnt on the back of his back.” — James Curran, FDNY
- “…and then we’re engulfed in the smoke, which was horrendous. One thing I remember, it was hot. The smoke was hot and that scared me.” — Paramedic, Manuel Delgado
- “I remember making it into the tunnel and it was this incredible amount of wind, debris, heat….” — Brian Fitzpatrick, FDNY Firefighter
- “A huge, huge blast of hot wind gusting and smoke and dust and all kinds of debris hit me.” — Firefighter, Louis Giaconelli
- “This super hot wind blew and it just got dark as night and you couldn’t breathe.” – Firefighter Todd Heaney
- “The whole block I think was on fire. All the parked cars were on fire. There were a couple of firemen hooked up right to a hydrant fighting the car fires.” — Firefighter, Peter Giammarino
As for the air emissions, many courageous people responded to the tragedy in New York by working to search for survivors, clean up the site, and get lower Manhattan back into working order. Thousands of these people have become sick and are dying from the exposure to that environment. The US government ignored them for many years but finally passed a bill last year providing limited medical support.
The unavoidable conclusion is that there is a great deal of evidence for the presence of unusually high temperatures at the WTC site on 9/11 and in the months afterward.
Endnotes
- Jones SE, Farrer J, Jenkins GS, et al. Extremely high temperatures during the World Trade Center destruction. Journal of 9/11 Studies 2008; 19: 1-11. [Accessed February 7, 2009]. Available from: http://www.journalof911studies.com/articles/WTCHighTemp2.pdf
- Ryan KR, Gourley JR, Jones SE. Environmental anomalies at the World Trade Center: evidence for energetic materials. Environmentalist 2009; 29(1):56-63. [Accessed February 7, 2009]. Available from: http://www.springerlink.com/content/f67q6272583h86n4
- Presentation by Leslie Robertson, http://www.youtube.com/watch?v=rjmHqES_lto
- Structural Fire Protection, ASCE Manuals and Reports on Engineering Practice no. 78,1992, p 172
- Kevin R. Ryan, Metallic Microspheres in WTC Dust, OpEd News, January 6, 2008. Available from: http://www.opednews.com/articles/life_a_kevin_ry_080106_metallic_microsphere.htm
- Paul J. Lioy, Dust: the inside story of its role in the September 11th aftermath, p 219
- RJ Lee Group, WTC Dust Signature Report, December, 2003.
- Ibid.
- Ibid.
- Federal Emergency Management Agency (FEMA), World Trade Center building performance study: Preliminary observations, and recommendations, Report FEMA 403. Washington, D.C.: Federal Emergency Management Agency, May 2002.
- James Glanz and Eric Lipton, A Search for Clues In Towers’ Collapse; Engineers Volunteer to Examine Steel Debris Taken to Scrapyards, The New York Times, February 2, 2002.
- Eric Lipton and Andrew C. Revkin, A NATION CHALLENGED: THE FIREFIGHTERS; With Water and Sweat, Fighting the Most Stubborn Fire, The New York Times, November 19, 2001.
- Garrett L, Full effects of WTC pollution may never be known. Newsday, 14 September 2003.
- Kidder M, Britt PF, Zhang Z et al, Pore size effects in the pyrolysis of 1,3-Diphenylpropane confined in mesoporous silicas. Chem Commun (Camb) 2003:2804–2805. doi:10.1039/b310405b.
- Cahill TA, Shackelford CJ, Meier M et al, Very fine aerosols from the World Trade Center collapse piles: anaerobic incineration. http://e-reports-ext.llnl.gov/pdf/305393.pdf. Accessed 16 Feb 2008
- Jim Hoffman, The North Tower’s Dust Cloud: Analysis of Energy Requirements for the Expansion of the Dust Cloud Following the Collapse of 1 World Trade Center, 911Research.wtc7.net, October 16, 2003



