Abstract: In an effort to better understand the conditions that led to complete collapses of the World Trade Center Towers and WTC 7, we apply scanning electron-microscope (SEM) and energy dispersive x-ray spectroscopy (XEDS) methods to analyze the dust generated, with an emphasis on observed micro-spheres in the WTC dust. The formation of molten spheres with high iron contents along with other species in the WTC dust required extremely high temperatures. Our results are compared with those of other laboratories. The temperatures required for the molten sphere-formation and evaporation of materials as observed in the WTC dust are significantly higher than temperatures associated with the burning of jet fuel and office materials in the WTC buildings.
1. Introduction
The events of 9/11/2001 were tragic and at the same time remarkable in their physical aspects, such as the completeness and rapidity of collapse of three skyscrapers and the large volume of fine toxic dust generated. In order to better understand these events, we obtained and examined two independent dust samples acquired very soon after 9/11/2001. The provenance of the two samples analyzed for this paper is described in the appendix. It is worth emphasizing that both of the samples were collected indoors and shortly after the 9/11/2001 event. One sample was collected on an indoor window sill on 9/14/2001, just three days after the disaster while searching for survivors in the rubble was ongoing, and in a building four blocks from ground zero. The other sample was acquired inside a fourth-floor apartment (whose upper windows broke during the WTC collapse) a few days later. We sought for samples acquired very soon after the collapses in order to drastically reduce any chance of contamination by clean-up operations (see Appendix). Furthermore, as we shall see, samples independently collected by other researchers corroborate the high-temperature indicators we observe.
2. Methods
A FEI XL30-SFEG scanning electron microscope (SEM) equipped with an EDAX Genesis X-ray energy dispersive spectrometry (XEDS) system was used to acquire XEDS spectra. A silicon detector (SiLi) with resolution better than 135 eV was used. The display resolution was set to 10 eV per channel. The operating conditions for the dust analyses were 20 keV, and 60-120 second acquisition time (livetime). The samples were analyzed at a 10 millimeter working distance and were mounted on carbon conductive tabs. Optical examination of the dust samples was conducted using a stereomicroscope (Nikon Epiphot 200) having a magnification range from 10-200X.
3. Results
We found an abundance of tiny solidified droplets roughly spherical in shape (spherules) in the WTC dust samples as shown in figure 1 (optical microscope) and figure 2 (Scanning Electron Microscope).


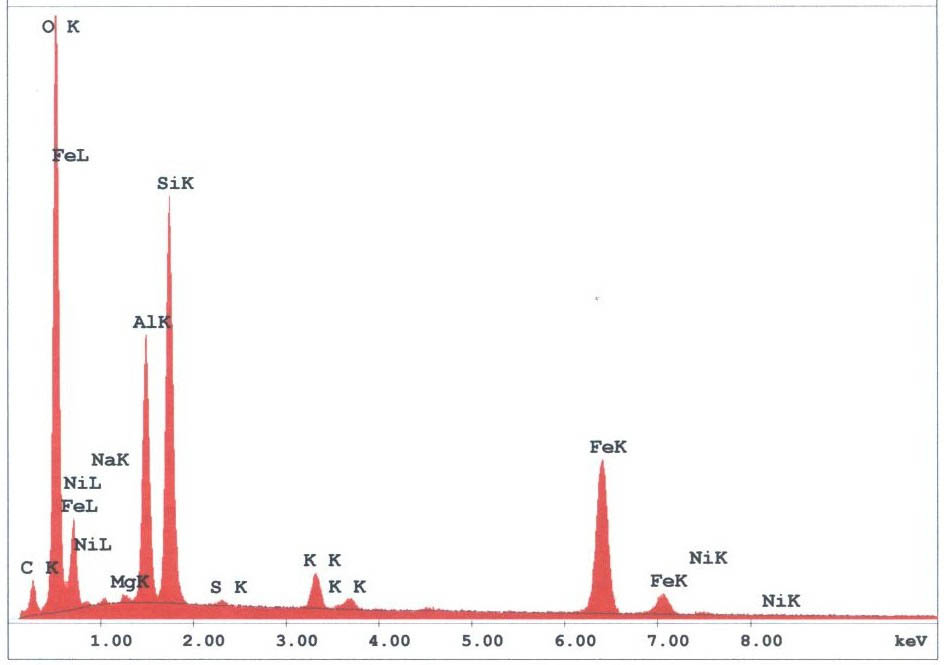
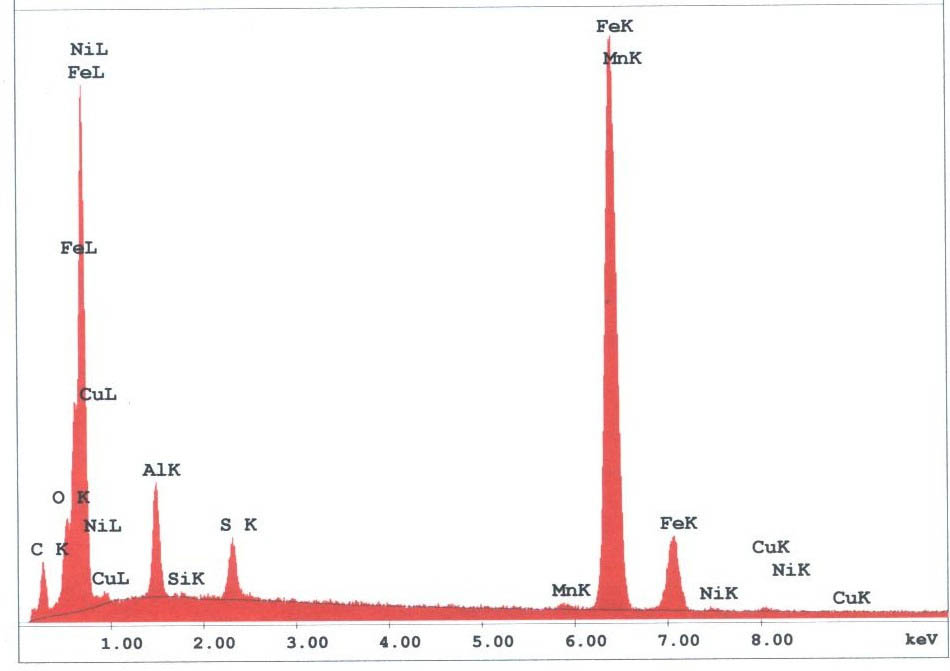
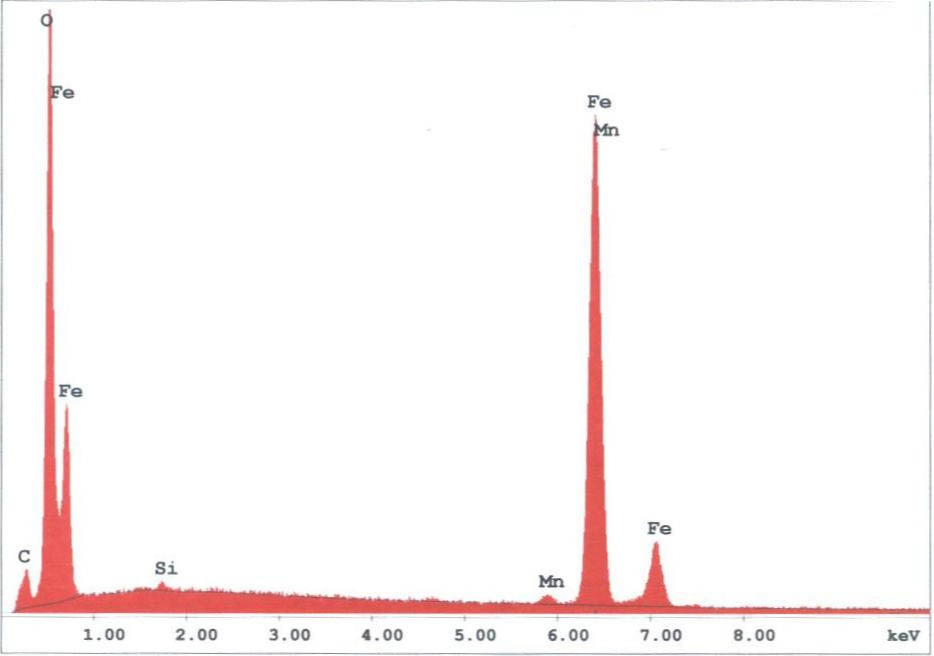
The spherules found in the WTC dust were predominately iron-rich (appearing metallic) and silicates(appearing glassy under an optical microscope). We observed spherules in a wide range of diameters, from about 1 micron to 1.5 mm. Figures 3 -5 provide X-ray energy dispersive spectra for observed iron-rich spherules from the WTC dust.
4. Discussion of relevant previously-published data
4.1. Observations of iron-rich and silicate spherules
Iron-rich spherules were also observed in studies conducted by the RJ Lee company [1] and the US Geological Survey (USGS) [2]. In particular, a USGS report on the WTC dust provides two micrographs of “iron-rich spheres” [3] and a “bulbous” or tear-drop-shaped silicate droplet [4] (see images below).

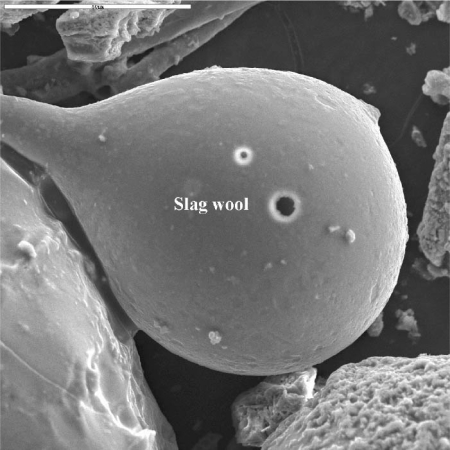
No explanation for the presence of these iron-rich and silicate spheres (which imply very high temperatures along with droplet formation) is given in the published USGS reports.
The RJ Lee report also provides a micrograph and XEDS data for iron-rich spheres observed in the WTC dust; for example, their figure 21 (below, left) shows an “SEM image and EDS of spherical iron particle [1].”We likewise observe high-iron, relatively low oxygen spheres (e.g., below right and Fig. 4), which we find are unlike spheres gathered from cutting structural steel with an oxyacetylene torch.
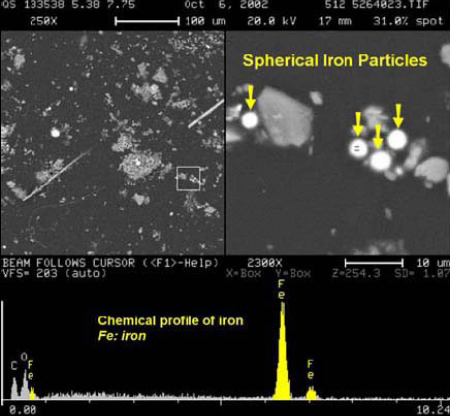
Moreover, the RJ Lee report provides provocative data regarding the abundance of observed iron-rich spheres. A WTC dust sample acquired at 130 Liberty Street shows a “mean of composition” of “Fe spheres” of 5.87% which is very high compared with “Fe spheres” found in ordinary building dust of only0.04% [1]. As the report notes, the WTC dust has unusual identifying characteristics – in particular, the WTC dust in this sample has nearly 150 times (5.87/0.04) the amount of iron-rich spheres as ordinary dust (where Fe spheres can arise from micrometeorites, for example).
There are other identifying features that characterize WTC dust compared with ordinary office dust; the RJ Lee report concludes:
“Various metals (most notably iron and lead) were melted during the WTC Event, producing spherical metallic particles. Exposure of phases to high heat results in the formation of spherical particles due to surface tension…”
“In addition to the vesicular carbon components, the high heat exposure of the WTC Dust has also created other morphologically specific varieties of particulate matter including spherical metallic, vesicular siliceous and spherical fly ash components. These types of particles are classic examples of high temperature or combustion by-products and are generally absent in typical office dust…”
“Particles of materials that had been modified by exposure to high temperature, such as spherical particles of iron and silicates, are common in WTC Dust because of the fire that accompanied the WTC Event, but are not common in “normal” interior office dust…”
“Combustion-related products are significant WTC Dust Markers, particularly if seen in combination [5].”
We agree with the RJ Lee report that the abundance of “spherical particles of iron and silicates” is proof of high temperatures, and that these particles are not common in normal office dust, but we do not agree that this abundance is necessarily due to the “fire that accompanied the WTC Event”. Before drawing such a conclusion, one must scrutinize the temperatures and other conditions needed to form these molten spheres (iron melts at 1,538 °C (2,800 °F) while iron (III) oxide melts at 1,565 °C (2,849 °F) [6] and aluminosilicates melt around 1,450 C [7]) and then compare with conditions reached in the WTC fires. We will turn to this task, after considering other data which also point to anomalously high temperatures during the WTC destruction.
4.2. Volatilized lead
The RJ Lee report notes “extremely high temperatures during the collapse which caused metallic lead to volatilize, oxidize, and finally condense on the surface of the mineral wool [1].” Again, “metals were vaporized at the WTC during the WTC Event and either deposited on WTC Dust or deposited directly onto surfaces in the Building [1].” Where do the requisite high temperatures come from?
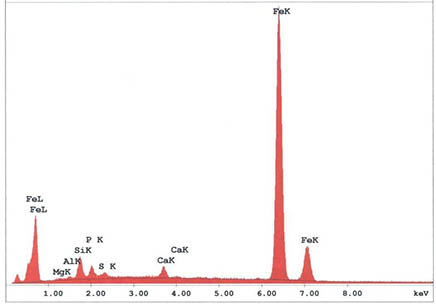
An additional characteristic of WTC Dust is the presence of coated particles and fibers. The coatings vary in thickness from monolayers to finely-dispersed sub-micron sized particles. The coated particles have been detected by low voltage back-scattered electron imaging, x-ray microprobe analysis, and high resolution x-ray photoelectron spectroscopy (XPS) as illustrated as an example in figure 3 and figure 4. Figure 3 shows traces of lead compounds identified on the surfaces of mineral wool by XPS, and the analysis of x-ray photoelectron spectra led to the identification of two peaks containing either lead oxide or lead sulfate (figure 4). The presence of lead oxide on the surface of mineral wool indicates the existence of extremely high temperatures during the collapse which caused metallic lead to volatilize, oxidize, and finally condense on the surface of the mineral wool [1].
The temperature required to volatilize/boil lead is 1,740 C or 3,164 F [8]. No explanation for the origin of the indicated “extremely high temperatures during the collapse” is offered in the RJ Lee report.
4.3. Molybdenum spherule in the USGS data set
Two of the authors pursued a Freedom of Information Act (FOIA) action with the USGS to obtain any additional SEM/XEDS data from them which had not been previously published. The new data demonstrated, significantly, that the USGS team had observed and studied a molybdenum-rich spherule which was not mentioned in the earlier reports. A micrograph image shows a bright pill-shaped spherule labeled “20MOSPH-1.TIF” (below). The brightness of the object suggests that backscattered electron imaging was used in acquiring the image (the report notes that this technique was used in the study), and that indeed a heavier metal such as Mo is present in the oblong spherule. (We see similar shapes; see SEM image below right and Fig. 1.)
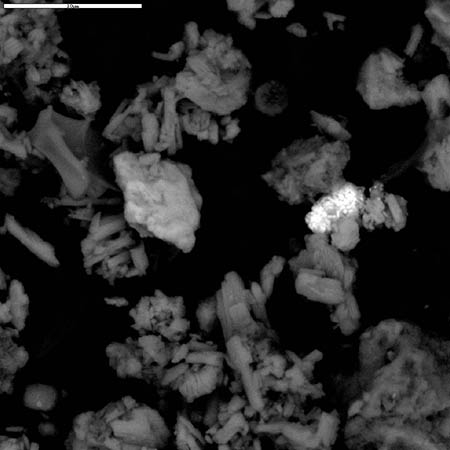
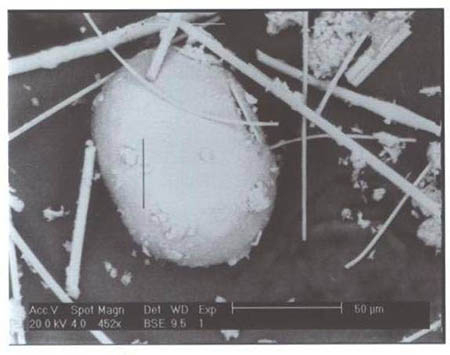
In the same data set obtained via FOIA action, an XEDS spectrum labeled “Molysph. TIF” and two nearly identical XEDS curves (with additional elemental identifications) labeled “Molly spectrum 25 kV” accompany what we deduce applies to the Mo spherule, given the designations. One of the spectra extends to approximately 19 keV (the electron beam energy was no doubt 25 kV), and shows a clear Mo line at 17.3 keV. This removes ambiguity regarding the peak at ~2.3 keV, which could otherwise be S or Pb or Bi (for example).The XEDS plots for this spherule labeled “Moly sph” show significant concentrations of aluminum, oxygen and calcium in addition to the dominant molybdenum peak and deserve further investigation. (For example, the Mo/Al peak height ratio is Mo:Al :: 5:1.)
We discern that considerable study was performed on this Mo-rich spherule, given the number of image sand XEDS plots for it, yet these data were not previously released in the public USGS reports.[2] We emphasize these data because of the extremely high melting temperature of molybdenum, and the observation of this molybdenum-rich spherule. Molybdenum is a refractory metal known for its extremely high melting point [9]. Mo melts at 2,623 °C (4,753 °F) [10], although addition of other elements may lower the melting point. No explanation of the high temperature needed to form the observed Mo-rich spherule is given in the USGS material (either published or obtained by FOIA action).
4.4. Materials from WTC with a “Swiss-cheese appearance” corroborate high temperatures
Dust particles from the WTC collapse show a “Swiss cheese appearance as a result of boiling and evaporation,” as reported in the RJ Lee report:
Additionally, WTC Dust can be differentiated from other building dust on the basis of its unique composition and morphology. WTC Dust Markers exhibit characteristics of particles that have undergone high stress and high temperature. Asbestos in the WTC Dust was reduced to thin bundle sand fibrils as opposed to the complex particles found in a building having asbestos-containing surfacing materials. Gypsum in the WTC Dust is finely pulverized to a degree not seen in other building debris. Mineral wool fibers have a short and fractured nature that can be attributed to the catastrophic collapse. Lead was present as ultra fine spherical particles. Some particles show evidence of being exposed toa conflagration such as spherical metals and silicates, and vesicular particles (round open porous structure having a Swiss cheese appearance as a result of boiling and evaporation).-Materials transformed by high temperature (burning). These transformed materials include: spherical iron particles, spherical and vesicular silicates, and vesicular carbonaceous particles. These heat-processed constituents are rarely, if ever, found together with mineral wool and gypsum in “typical” indoor dusts [1].
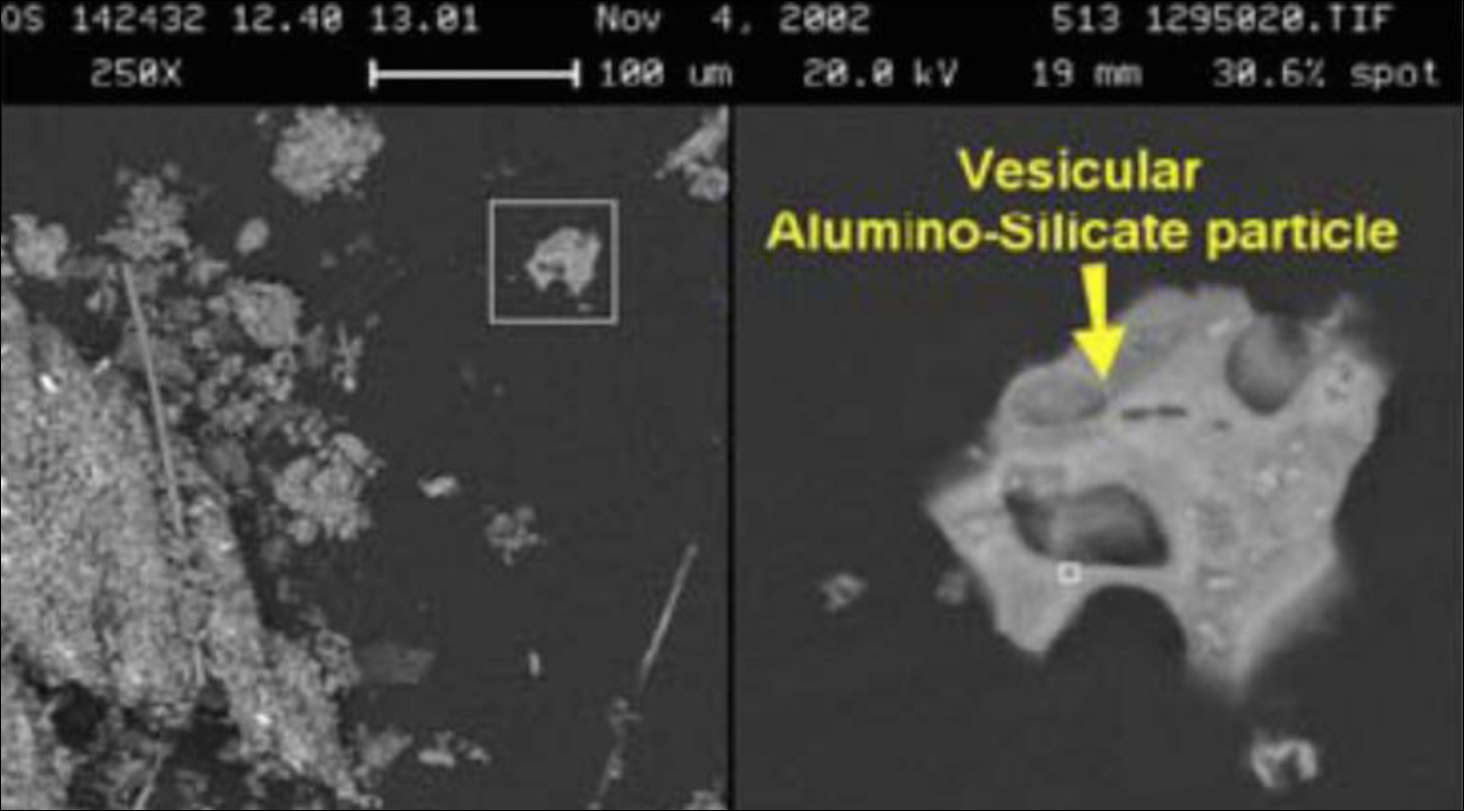
The RJ Lee report provides an image of a “vesicular alumino-silicate particle” which exemplifies the “round open porous structure having a Swiss cheese appearance as a result of boiling and evaporation”(below, left). [1] The images below “show the difference between an angular non-porous non-heat affected particle within a Background Building [right] and a porous WTC Dust silicate heat-affected particle [left]”.
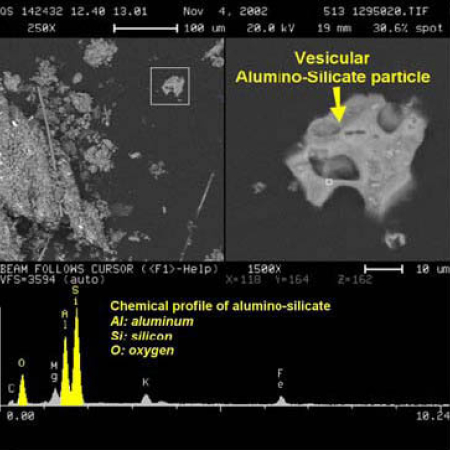
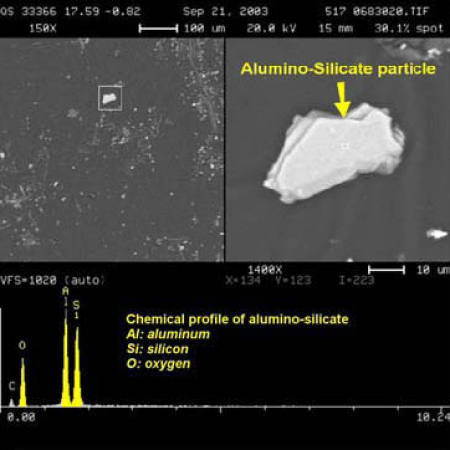
It is not clear to us that boiling of aluminosilicate is needed to produce the observed porous structure; melting and evaporation of some minor component may suffice. But if the “Swiss-cheese appearance” is indeed a result of “boiling and evaporation” of the material as the report suggests [1], we note the boiling temperature for aluminosilicate is approximately 2,760 °C [11].
The phrase “Swiss cheese appearance” was also used by a team from Worcester Polytechnic Institute led by Prof. Jonathon Barnett in describing steel from WTC 7.
The significance of the work on a [steel] sample from Building 7 and a structural column from one of the twin towers becomes apparent only when one sees these heavy chunks of damaged metal. A one-inch [steel] column has been reduced to half-inch thickness. Its edges–which are curled like a paper scroll–have been thinned to almost razor sharpness. Gaping holes–some larger than a silver dollar–let light shine through a formerly solid steel flange. This Swiss cheese appearance shocked all of the fire-wise professors, who expected to see distortion and bending–but not holes [12].
The researchers argue that a eutectic formed at approximately 1,000 °C in this steel sample from WTC 7:
A liquid eutectic mixture containing primarily iron, oxygen, and sulfur formed during this hot corrosion attack on the steel… The eutectic temperature for this mixture strongly suggests that the temperatures in this region of the steel beam approached 1,000 °C (1,800 °F), which is substantially lower than would be expected for melting this steel [13].
However, to form a molten iron-oxygen-sulfur eutectic at about 1,000 °C would require a very high concentration of sulfur, around 50 (mol. %) [14]. ]. The fact that sulfur evaporates at a low temperature, 445°C, along with the very low levels of elemental sulfur in office buildings appears to preclude the possibility that the eutectic could have formed as a result of a slow sulfidation process in the debris pile. In any case, the authors admit that
The severe corrosion and subsequent erosion of Samples 1 [WTC7] and 2 [Towers] are a very unusual event. No clear explanation for the source of the sulfur has been identified [13].
Nevertheless, we will provisionally accept their analysis that a steel (not air) temperature of about 1,000 °C was reached, pending understanding of the source of the observed sulfur. (The XEDS plots shown by the authors show sulfur without concomitant calcium, which would be present for a calcium-sulfate (gypsum)contamination.) [13] The other data summarized here point to significantly higher temperatures than 1,000 °C.
It is interesting that the FEMA report discussed the “evidence of a severe high temperature corrosion attackon the steel, including oxidation and sulfidation” and called for further investigation, [13] – but the subsequent NIST report [15] failed to address this evidence. Nor did NIST address the published observations of abundantiron-rich spherules in the WTC dust [1, 2]. We find that these effects are important to understanding the events of 9/11/2001 and should not be neglected.
4.5. Summary of Temperatures Required by the WTC Data
The formation of spherules in the dust implies the generation of materials somehow sprayed into the air so that surface tension draws the molten droplets into near-spherical shapes. That shape is retained as the droplet solidifies in the air. Spherules observed in the WTC dust include iron-rich, molybdenum-rich and silicate varieties. The temperatures required to melt iron, silicates, and molybdenum, and to vaporize lead and aluminosilicates (as discussed above) are summarized in table 1.
Table 1. Approximate Minimum Temperatures Required
| Process and material | °C | °F |
| To form Fe-O-S eutectic (with ~50 Mol % sulfur)in steel | 1,000 | 1,832 |
| To melt aluminosilicates (spherule formation) | 1,450 | 2,652 |
| To melt iron (spherule formation) | 1,538 | 2,800 |
| To melt iron (III) oxide (spherule formation) | 1,565 | 2,849 |
| To vaporize lead | 1,740 | 3,164 |
| To melt molybdenum (spherule formation) | 2,623 | 4,753 |
| To vaporize aluminosilicates | 2,760 | 5,000 |
4.6 Maximum temperatures associated with the WTC fires
Finally, we consider the temperatures reached in normal building fires, jet-fuel fires and in the World Trade Center buildings. Maximum temperatures due to fires in the WTC of around 1,000 C are argued by Thomas Eagar:
The fire is the most misunderstood part of the WTC collapse. Even today, the media report (and many scientists believe) that the steel melted. It is argued that the jet fuel burns very hot, especially with so much fuel present. This is not true…. The temperature of the fire at the WTC was not unusual, and it was most definitely not capable of melting steel.
In combustion science, there are three basic types of flames, namely, a jet burner, a pre-mixed flame, and a diffuse flame…. In a diffuse flame, the fuel and the oxidant are not mixed before ignition, but flow together in an uncontrolled manner and combust when the fuel/oxidant ratios reach values within the flammable range. A fireplace is a diffuse flame burning in air, as was the WTC fire. Diffuse flames generate the lowest heat intensities of the three flame types… The maximum flame temperature increase for burning hydrocarbons (jet fuel) in air is, thus, about 1000 °C — hardly sufficient to melt steel at 1500 °C.
But it is very difficult to reach this maximum temperature with a diffuse flame. There is nothing to ensure that the fuel and air in a diffuse flame are mixed in the best ratio… This is why the temperatures in a residential fire are usually in the 500 °C to 650 °C range. It is known that the WTC fire was a fuel-rich, diffuse flame as evidenced by the copious black smoke [16].”
NIST provides a maximum gas temperature due to WTC fires of 1,000 °C:
In no instance did NIST report that steel in the WTC towers melted due to the fires. The melting point of steel is about 1,500 degrees Celsius (2,800 degrees Fahrenheit). Normal building firesand hydrocarbon (e.g., jet fuel) fires generate temperatures up to about 1,100 degrees Celsius (2,000degrees Fahrenheit). NIST reported maximum upper layer air temperatures of about 1,000 degrees Celsius (1,800 degrees Fahrenheit) in the WTC towers (for example, see NCSTAR 1, figure 6-36) [17].
Based on this comprehensive investigation, NIST concluded that the WTC towers collapsed because: (1) the impact of the planes severed and damaged support columns, dislodged fireproofing insulation coating the steel floor trusses and steel columns, and widely dispersed jet fuel over multiple floors; and (2) the subsequent unusually large jet-fuel ignited multi-floor fires (which reached temperatures as high as 1,000 degrees Celsius) significantly weakened the floors and columns with dislodged fireproofing to the point where floors sagged and pulled inward on the perimeter columns[18].
In actual metallurgical analyses of WTC steel, NIST reports:
These [steel] microstructures show no evidence of exposure to temperatures above 600°C for any significant time [18].
In a report entitled “Fire Safety in High-rise Buildings, Lessons Learned from the WTC,” a team of fire experts notes:
Standard structural fire testing exposes elements to about 900 °C in 1 hour and up to 1,100 °C by 4hours. The expectation of these standard tests could well be defined as providing a worst-case fire scenario [19].
All of these estimates for the WTC fires (including burning jet fuel) put the temperature well below the melting point of steel, about 1,500 °C [17]. In fact, the non-melting of WTC steel is emphasized by NIST – but they fail to address the presence of large numbers of iron-rich spherules in the dust published in USGS and other reports before the NIST study was published in October 2006 [16, 18].
Table 2 summarizes maximum temperatures determined for the WTC fires.
Table 2. Maximum Gas Temperature Reached in WTC Fires [20]
| Analysis | °C | °F |
| Thomas Eager analysis | 1,000 | 1,832 |
| NIST analysis | 1,000 | 1,832 |
| Torero, Quintiere, Steinhaus | 1,100 | 2,012 |
5. Sphere formation and size
Not only is it necessary for the material to have achieved extremely high temperatures to melt and so be able to form small spheres, it is also necessary that some violent physical disturbance occur in order to shatter the molten material into the sizes observed, 1.5mm down to about one micron diameter. Then surface tension in the liquid droplets brings about spherule formation. Various explosive chemical reactions will (for example)result in formation of spherules in the end products. The NIST report states that “no evidence” for explosives was found [15] but it is clear from these data that this issue should be addressed again.
6. Discussion
The temperatures required for the observed spherule-formation and evaporation of materials observed in the WTC dust (table 1) are significantly higher than temperatures reachable by the burning of jet fuel and office materials in the WTC buildings (table 2). The temperatures required to melt iron (1,538 °C) and molybdenum(2,623 °C), and to vaporize lead (1,740 °C) and aluminosilicates (~2,760°C), are completely out of reach of the fires in the WTC buildings (maximum 1,100 °C). We wish to call attention to this discrepancy: the official view implicating fires as the main cause for the ultimate collapses of the WTC Towers and WTC 7 (FEMA[13], NIST [15] ) is inadequate to explain this temperature gap and is therefore incomplete at best. The formation of numerous metal-rich spherules is also remarkable, for it implies formation of high-temperature droplets of the molten metals, dispersed in the air where they cool to form spherules. As displayed in figures 3and 4, we observe spherules with high iron and aluminum contents, a chemical signature which is not consistent with formation from melted steel.
The data provide strong evidence that chemical reactions which were both violent and highly-exothermic contributed to the destruction of the WTC buildings. NIST neglected the high-temperature and fragmentation evidence presented here: it appears nowhere in their final report [15]. Proposed new building codes based on the WTC disaster must address all available evidence for what caused the complete and rapid destruction of these skyscrapers. Understanding the mechanisms that led to the destruction of the World Trade Center will enable scientists and engineers to provide a safer environment for people using similar buildings and benefit firefighters who risk their lives trying to save others. Thus, a thorough investigation which considers these data, showing extremely high temperatures and severe fragmentation in the formation of small metal-rich spheres during the WTC Towers destruction, is highly motivated. In particular, the repeatedly-delayed report on the destruction of WTC 7 on 9/11/2001 [21] should address these striking facts.
Appendix
Provenance of dust samples analyzed in original work reported here.
Sample 1 was collected from inside the Potter Building located at 38 Park Row in New York City. It was collected by a Ph.D. scientist on 9/14/2001, just three days after the 9/11/2001 and before any major steel-cutting operations had begun at ground zero. Rescue operations were on-going at the time of sample collection. Furthermore, the building is located about four blocks from ground zero and the sample was collected from dust that had worked its way inside the building, landing on an interior window sill. Thus, contamination from steel-cutting operations at ground zero (which can produce molten steel spheres) can be ruled out with a very high degree of confidence. The iron-rich spheres collected in sample 1 are evidence of high-temperature melting and violent fragmentation during the WTC destruction and dust formation.
Sample 2 was collected by Jeannette MacKinlay about a week after 9/11/2001, from inside her apartment at113 Cedar St./110 Liberty St., New York City. WTC dust entered her apartment through two windows which broke as the South Tower collapsed. The holes in the windows were approximately 0.5 m X 0.8 m, and the apartment was on the fourth floor.
In both samples, elements besides iron are often present in the spheres which yield chemical signatures distinct from that of structural steel (such as Al, Si, Cu, K, S; see Figs. 3 and 4). These chemical signatures provide additional evidence that the spheres did not result from steel-cutting operations during clean-up. We have recently obtained a WTC dust sample acquired within twenty minutes of the collapse of the North Tower, near the Brooklyn Bridge, which also shows spherules like those shown in Figs. 1-5. These spheres cannot have originated from the later clean-up operations. Further results from our on-going investigation will be presented in future papers. Probing alternative chemical reactions which could have produced these spherules is beyond the scope of this paper; but further analyses of these contaminants may provide important clues regarding the processes which generated the observed iron-rich spheres and concomitant high temperatures.
References
- RJ Lee Group, WTC Dust Signature Report, December, 2003, available here:
http://www.nyenvirolaw.org/WTC/130%20Liberty%20Street/Mike%20Davis%20LMDC%20130%20Liberty%20Documents/Signature%20of%20WTC%20dust/WTC%20Dust%20Signature.Composition%20and%20Morphology.Final.pdf; and
http://www.nyenvirolaw.org/WTC/130%20Liberty%20Street/Mike%20Davis%20LMDC%20130%20Liberty%20Documents/Signature%20of%20WTC%20dust/WTCDustSignature_ExpertReport.051304.1646.mp.pdf - Heather A. Lowers and Gregory P. Meeker . Particle Atlas of World Trade Center Dust, available here:
http://pubs.usgs.gov/of/2005/1165/508OF05-1165.html - http://pubs.usgs.gov/of/2005/1165/graphics/IRON-04-IMAGE.jpg, and http://pubs.usgs.gov/of/2005/1165/graphics/IRON-03-IMAGE.jpg.
- http://pubs.usgs.gov/of/2005/1165/graphics/SLAGWOOL-03-IMAGE.jpg.
- RJ Lee Group, WTC Dust Signature Report, Composition and Morphology, December, 2003, Table 3.Note that this sample was acquired close to the WTC complex and thus may have more of the dense Fe-spherecontent than dust samples acquired at greater distances.
- http://www.webelements.com/webelements/elements/text/Fe/heat.html, http://physchem.ox.ac.uk/MSDS/IR/iron_III_oxide.html.
- http://ceramic-materials.com/cermat/material/2165.html
- http://www.chemicalelements.com/elements/pb.html
- http://www.rembar.com/elements.html
- http://www.webelements.com/webelements/elements/text/Mo/heat.html
- http://chemicalland21.com/industrialchem/inorganic/BENTONITE.html
- (“The “Deep Mystery” of Melted Steel,” WPI Transformations, Spring 2002,
http://www.wpi.edu/News/Transformations/2002Spring/steel.html) - http://www.fema.gov/library/wtcstudy.shtm , esp. Appendix C,
http://www.fema.gov/pdf/library/fema403_apc.pdf - P. Asanti and E. J. Kohlmeyer, Z. Anorg. Chem., 265:94 (1951).
- National Institute of Standards and Technology (NIST). (2005). “Final Report on the Collapse of the World
Trade Center Towers.” S. Shyam Sunder, et al. (available at http://wtc.nist.gov) - Eagar, T. W. and Musso, C. (2001). “Why Did the World Trade Center Collapse? Science, Engineering,
and Speculation”, Journal of the Minerals, Metals and Materials Society, 53/12:8-11 (2001). - http://wtc.nist.gov/pubs/factsheets/faqs_8_2006.html
- See NIST Report, NCSTAR 1-3, p. xli (emphasis added)
- J.L. Torero, J.G. Quintiere and T. Steinhaus, “Fire Safety in High-rise Buildings: Lessons Learned from the
WTC,” Jahresfachtagung der Vereingung zur Forderrung des Deutschen Brandschutzez e. V., Dresden,
Germany, 2002. See also, James Quintiere, “Questions on the WTC Investigations,” World Fire Safety
Conference, June 2007. - Even in a testing furnace when the air temperature is raised quickly and held at 1,000 °C, and heat is not
conducted away by a large building structure, it takes approximately two hours for protected steel to reach 600
°C. (Structural Fire Protection, ASCE Manuals and Reports on Engineering Practice no. 78,1992, p 172.) - http://www.nist.gov/public_affairs/releases/wtc_062907.html
Endnotes, 2/7/08: USGS = US Geological Survey. (SiLi) detector. In Figs 3-5, elemental atomic % as given by theEDAX system including analysis package; to test system consistency, we made eight 50-second measurements onFe2O3 samples and found consistency for iron ± 6.2% and for oxygen ± 3.4% (statistical, 1 sigma; see Fig. 5).